Aadhaar eKYC Advantages: Speed, Savings, and Security | The Digital Transformation
In the digital age, identity verification is a crucial component of countless services and transactions. Aadhaar eKYC (Electronic Know Your Customer)

Selecting the Perfect Aadhaar eSignature Vendor: A Comprehensive Guide
Aadhaar-based eSignatures have become an integral part of India’s digital landscape, revolutionizing how businesses and individuals authenticate

eSign Audit Trails: Your Digital Signature Safety Net
In today’s digitally-driven world, electronic signatures, commonly known as eSignatures, have transformed the way we sign and exchange documents

eSign Solutions for NGOs: Supercharge Donations & Fundraising
In the world of non-governmental organizations (NGOs), the ability to efficiently manage donations and fundraising is crucial.

Close Deals Faster with eSignatures: A Comprehensive Guide for Sales Teams
In today’s fast-paced business environment, time is money. For sales teams, closing deals quickly can make the difference between success and st

Comprehensive Guide to eSign Key Fact Statements (KFS) with Digital Signatures
In today’s digital age, the way we handle documents has undergone a significant transformation.

Unlocking the Power of Aadhaar eSign API: Your Guide to Effortless eSignatures
In today’s fast-paced digital world, electronic signatures have transitioned from being a luxury to a necessity.
MeiTY IT Act Amendment Empowered Five Document Types
In today’s digital era, electronic signatures (e-signatures) have emerged as a powerful tool, transforming the way we handle official documents.

eSignatures in Real Estate: Embracing Efficiency with the IT Act Amendment
In a rapidly evolving digital age, real estate transactions are no exception to the transformative power of technology.

Mastering Online Signatures: A Comprehensive Guide for Businesses
In today’s digital age, the business world is constantly evolving, and technological advancements are transforming the way we conduct business.

Sign with Confidence: A Global Guide to E-Signature Laws
In today’s digital age, the world has witnessed a profound transformation in the way businesses operate.

How to Sign Contracts Online | MSB Docs
In today’s digital age, almost every aspect of business and personal life has been transformed by technology.

Unlocking the Power of Digital Contracts: Legal Validity and Enhanced Security
In the digital age, the business landscape is undergoing a profound transformation.

Mastering Online PDF Signatures: A Step-by-Step Guide for Legal, Secure, and Efficient Signing
In today’s digital world, the need to sign documents securely and efficiently is paramount.

Secure Healthcare Documents with HIPAA Compliant E-Signature Solutions | MSB Docs
In today’s digital age, the healthcare industry faces numerous challenges, and one of the most pressing concerns is ensuring the security and in

Strengthening Online Banking Security: How KYC Fights Identity Theft and Fraud
In today’s digital age, online banking and financial transactions have become an integral part of our lives.

Streamline Vendor Contracts & Onboarding: Automation with MSB Docs
Vendor contracts and onboarding play pivotal roles in managing business relationships with suppliers and service providers.

Optimizing Credit Card Onboarding with Video KYC: Streamlining the Process
Credit card onboarding, a crucial process in the financial industry, can often be a source of frustration and complexity for both financial institutio

Revamp Your KYC Process for Business Success: 3 Key Changes to Embrace
In today’s rapidly evolving business landscape, one thing remains constant: the importance of the Know Your Customer (KYC) process.
Document Automation Reality Check: How MSB Docs eSignature Delivers Results
In today’s digital age, businesses across various industries are constantly seeking ways to streamline their operations and enhance efficiency.

Revolutionizing FMCG Vendor Onboarding with MSB Docs eSignature: Streamlined Efficiency
The Fast-Moving Consumer Goods (FMCG) industry is a highly competitive and dynamic sector that relies on efficient supply chain management.

Unlocking Efficiency: eSignatures in Franchise Agreements | MSB Docs
In the ever-evolving landscape of business operations, the traditional processes of handling contracts and agreements are rapidly giving way to digita

“Unlocking Efficiency and Security: The Benefits of eKYC for Customer Onboarding”
In today’s fast-paced digital world, customer onboarding is a critical process for businesses across various industries.

“Transforming MSME Onboarding: How Video KYC Drives Efficiency and Growth”
Micro, Small, and Medium Enterprises (MSMEs) are the lifeblood of many economies, contributing significantly to employment and economic growth.
7 Fascinating Facts About Video KYC and the Role of MSB Docs eKYC Solution
In today’s fast-paced digital world, businesses are constantly seeking innovative solutions to streamline their operations, enhance security, an

Guarding Against Identity Theft in Online KYC: Tips and Solutions
In today’s digital age, online video KYC (Know Your Customer) has become a crucial component of identity verification processes for businesses a

Elevating Retail & FMCG Security: The Power of Vendor KYC Verification
In the ever-evolving landscape of retail and Fast-Moving Consumer Goods (FMCG), the efficient procurement and distribution of products are critical fo

Achieving Business Compliance: A Deep Dive into KYC Practices | MSB Docs
In today’s global business landscape, where transactions cross borders and industries seamlessly, the need for robust compliance measures has ne

Achieve Total Quality Mgmt – Complete Guide to ISO 13485 Certification
ISO 13485 certification is an internationally recognized set of standards aimed at ensuring that medical device manufacturers have implemented stringe

Unveiled: A Guide to What is FDA 21 CFR Part 111?
The Food and Drug Administration (FDA) created 21 CFR Part 111 in 2007 for the purpose of regulating dietary supplements.

Uncovering the Regulator: Exploring the Therapeutic Goods Administration (TGA)
The Therapeutic Goods Administration (TGA) is the Australian Government agency responsible for regulating therapeutic goods including medicines, medic
Uncover the Benefits & Challenges of Clinical Laboratory Improvement Amendments (CLIA).
Clinical Laboratory Improvement Amendments (CLIA) is a set of laws and regulations that are designed to ensure that all laboratory testing is performe

Discover What is ISO/TS 16949 & IATF 16949 & Why it Matters
ISO/TS 16949 and IATF 16949 are two important standards recognized globally for automotive-specific Quality Management Systems (QMS).
Transition Your Medical Devices to New European MDR: Are You Ready?
Welcome to our guide on transitioning your medical devices to Europe’s New Medical Device Regulation (MDR)! This guide will provide an overview

What is 21 CFR Part 606? An Essential Guide to FDA Compliance
The FDA 21 CFR Part 606 regulations are designed to regulate the safety of both food and drug products.

Unpacking the Regulations: Understand 21 CFR 1271
21 CFR 1271 is a regulation set forth by the US Department of Health and Human Services (HHS) that addresses the manufacturing, processing, and labeli

Unveiling the Mysteries of 21 CFR Part 820 & ISO 13485
The 21 CFR Part 820 is a quality system regulation (QSR) issued by the United States Food and Drug Administration (FDA) which governs the design, manu

21 CFR Part 11: Open vs Closed Systems – What’s the Difference?
The 21st Century Cures Act (21 CFR Part 11) is a US Food and Drug Administration (FDA) regulation on the security and integrity of electronic records

Unraveling Regulations: 21 CFR Part 11 vs EU Annex 11
In today’s digital age, organizations must be equipped to manage electronic records in a consistent, secure manner. In order to achieve this obj

Compliance with HITRUST: What It Is and Why It Matters
HITRUST certification is a highly-regarded IT security and compliance certification program. HITRUST stands for Health Information Trust Alliance, and

Unlocking Healthtech Success:Understanding Business Models
When it comes to the world of healthcare, technology is becoming increasingly essential for medical organizations to increase patient satisfaction, re

Discover What Healthcare Compliance Means & How to Implement It
Healthcare compliance is the practice of making sure that organizations follow all laws, regulations, and policies related to the healthcare industry.

Unlock Digital Lending: Here’s How Banks Can Help
Digital lending is revolutionizing the way banks do business. It’s enabling banks to offer paperless loans to customers within a matter of minutes a

RBI Revamps Digital Lending: DLG Permitted to Streamline Loans
With more and more of our lives transitioning to the digital space, financial services are no exception. In an increasingly globalised and data-driven

Get the Lowdown on Affidavits + their E-Stamping Requirements
Affidavits are important documents that are sworn in writing and may be used as legal evidence in court.

Get the Basics of an NDA: 15 Questions to Ask Before Signing
The purpose of this guide is to provide readers with an understanding of Non-Disclosure Agreements (NDAs) and the 15 key questions they should ask the

Make an Impression: Unlock Your Unique Signature
A signature is a mark or symbol representing you. It is a unique, personal identifier that is attached to your identity to show ownership or to certif

Get more Secure: Learn Difference of E-Signature & Digital Signature
An electronic signature and a digital signature are two terms that are sometimes used interchangeably, but there is an important difference between th
What’s The Difference? Get the Lowdown on Agreements vs Contracts
Agreements and contracts are essential elements of our everyday lives, playing a crucial role in the way we conduct business and make commitments.

Master the Basics: Unlocking the Power of eMandates
An eMandate is an electronic authorization for direct debit payments. The system is designed to streamline the payment process, reducing administrativ

Take Control: Understand Breach of Contract to Avoid Disputes
Contracts are an essential part of doing business in the modern world. They provide a legal framework that defines the rights and obligations of each

Unlock the Digital Age: Uncover the Power of US Electronic Signature UETA & ESIGN
The Electronic Signature in Global and National Commerce Act (ESIGN) and the Uniform Electronic Transactions Act (UETA) are two pieces of legislation

Unveiling the Mystery of MGAs: The Ultimate Insurance Agent
An MGA, or Managing General Agent, is an important part of the insurance industry. They have various roles and responsibilities including finding comp

Conquer the Top 6 Quality & Compliance Oversights in Pharma
The success of any business in the pharmaceutical industry relies heavily on its ability to navigate the ever-changing regulations that are required f

Unlock HIPAA Compliance: The Complete Guide
The Health Insurance Portability and Accountability Act (HIPAA) is a standard which all healthcare providers, organizations, and insurance companies m

How to Write Perfectly for CAPA: An Essential Guide
CAPA (Corrective Action/Preventative Action) processes are essential requirements for many businesses in today’s world.

Solve Investigations Right: 5 Essential Mistakes To Avoid
Are you ready to get started on your investigation but don’t know where to begin? Writing an investigation often comes with its own set of challenge

Don’t Make These 5 Mistakes When Preparing an IND Filing Plan
Biotech startups are revolutionizing the medical and healthcare industry. From finding new ways to treat illnesses to developing innovative technologi

USP & FDA to Make Critical Updates to Good Storage & Distribution Practices
This blog post will discuss the proposed updates to Good Storage and Distribution Practices from the United States Pharmacopeia (USP) and the Food and

Equip Your Drug Launch with Nitrosamine Regulations: Essential Guide
This guide is designed to help organizations understand and comply with the nitrosamine regulations related to marketing applications for new drug pro

Need to Respond to FDA 483s? Here’s What You Need to Know
FDA 483 observations (or “Form 483s”) are issued by the Food and Drug Administration (FDA) when findings of a GMP (Good Manufacturing Prac

Learn to Uncover Value from Clinical Trials in Australia
Australia is home to world-class medical research, and clinical trials are a major part of this. Clinical trials are federally regulated studies that

Transform Healthcare: Learn How AI & Automation are Changing Medicine
With the introduction of Artificial Intelligence (AI) and automation, the medical information industry is undergoing a radical transformation.

Unlock the Potential of Clinical Research: Why Decentralized Clinical Trials Are the Future
Decentralized clinical trials (DCTs) are a new approach to conducting clinical research that shifts the traditional model in significant ways.

Discover What GxP Compliance Audits Are & Who Performs Them
GxP independent compliance audits are processes developed to ensure that companies adhere to certain standards of quality, safety, and regulatory comp

Ready for Clinical Trial GCP Audits? Here’s What You Need to Know
A Good Clinical Practice (GCP) audit is an important part of clinical trials that ensures that the methods used and data collected are meeting certain

An In-depth Look at Integrity & Reliability in Bioequivalence Studies
Bioequivalence studies are an important process for ensuring that the pharmaceutical products produced by drug companies meet regulatory standards, su

FDA Announces New Reg. for Easy-to-Understand Medication Info for Patients
When it comes to taking medications, it’s important to understand what you’re consuming and the possible risks associated with doing so.

Get Ready for the International Recognition Framework (IRF): Understand Your Commitments
The International Recognition Framework (IRF) is an important tool for organizations as it sets out requirements for compliance with international sta

Take a Peek Into Common Critical Findings Found in Compliance Auditing
Compliance auditing is an important component of regulatory compliance. It involves a thorough review of procedures, processes, and records to ensure

Don’t Miss Out: 5 Essential Mistakes to Avoid When Writing a Biotech Startup’s Development Plan
An Investigational New Drug (IND) application is a legal document submitted to the Food and Drug Administration (FDA).

Unlock Safety and Security with GVP: A Quick Guide
Good pharmacovigilance practices (GVPs) are procedures used to protect the safety of patients taking medications.

Uncovering FDA’s Nitrosamine Impurity Regulations for NDSRIs
NDSRI stands for Non-selective Dopamine Reuptake Inhibitor and is a type of medication used to treat depression and other mental health conditions.

Discover What the Drug Supply Chain Security Act (DSCSA) Means for You!
The Drug Supply Chain Security Act (DSCSA) is a law of the United States that was passed in 2013 to create a national system for the regulation and co

Capture the Benefits: Transitioning from Early-Stage to Late-Stage Biotech
Biotechnology is one of the most transformative forces in the world today. It stands poised to revolutionize our understanding of science, medicine, a

Grasp the Difference: Contract Management vs Contract Lifecycle Management
Contracts are a foundational element of any successful business. As such, it’s essential to understand the differences between contract management

Get Smart on KYC: What it Means & Why It Matters
KYC stands for Know Your Customer. It is a process that businesses use to verify the identity of their customers when they sign up for a product or se

Uncover the Must-Have KYC Checklist for a Compliant Program
Having a compliant Know Your Customer (KYC) program is essential for any business, no matter its size or sector.

Discover What Contract Lifecycle Management Is & How It Benefits Businesses
Contract Lifecycle Management (CLM) is a process that helps businesses effectively manage, control and organize the different stages of a contract’s

Don’t Miss Out: The Best Medical Events in 2nd Half of ’23
Medical device events are an important part of the larger medical device industry. Attending these events can help you to stay up-to-date with the lat

Unlock Science: How to Leverage NLP in the Life Sciences
Natural language processing (NLP) has revolutionized many industries, making complex processes easier and more efficient.

Unlocking AML Guidelines: IRDAI AML/CFT Compliance in Insurance
In the ever-evolving landscape of the insurance industry, staying compliant with regulatory guidelines is paramount.

VIPV KYC: A Comprehensive Guide to Video In Person Verification
In the ever-evolving realm of finance and digital transactions, ensuring the identity and legitimacy of customers is paramount.

Money Laundering in Insurance: Risks, Regulations, and Rewards | MSB Docs
Money laundering is a global menace that affects various sectors, including the insurance industry.

Demystifying SEBI’s 2023 Cloud Framework Guidelines
In the rapidly evolving landscape of financial markets, the Securities and Exchange Board of India (SEBI) stands as a formidable guardian of inte

Critical Assessment: RBI’s cKYC High-Risk Designation & the Path to Video KYC Adoption
In the world of financial services, the Know Your Customer (KYC) process stands as a guardian of integrity, transparency, and regulatory complian

RBI’s Latest Regulations: Safeguarding Digital Lending Against Malpractices
In today’s fast-paced digital age, the financial landscape is undergoing a significant transformation.

Unlocking Efficiency: How Digital Stamping Transforms Loan Disbursement for NBFCs
In the rapidly evolving landscape of financial services, Non-Banking Financial Companies (NBFCs) are playing a pivotal role in providing loans

Transforming Business: 5 Critical Industries Embracing Digital Stamp Solutions
In our rapidly evolving digital landscape, the importance of secure and efficient document authentication cannot be overstated.

E-Stamping: Your Complete Guide to Online Document Authentication
In today’s fast-paced digital world, the way we handle documents and transactions is constantly evolving.

Demystifying Differential Stamp Duty in India: Your Comprehensive Guide
Stamp duty is an essential part of the financial landscape in India, serving as a significant source of revenue for state governments.

Digital Stamping 101: How Banks Are Revolutionizing Document Workflow
In today’s fast-paced digital age, the banking industry is continually evolving to meet the needs of both customers and regulatory authorities.

Digital Stamping Revolution: Efficiency, Security, and Benefits for Businesses
In the digital age, businesses are constantly seeking innovative solutions to streamline their operations and improve efficiency.

Mastering Online Stamp Duty Payment: A 5-Step Guide for Effortless Transactions
Stamp duty is a crucial part of various financial transactions, whether it’s purchasing property, transferring shares, or executing legal d

E-Stamping in India 2023: A Comprehensive Guide to Digital Stamp Duty Payment
E-Stamping, short for electronic stamping, is a digital method of paying stamp duty for various legal documents and transactions.
Revolutionizing Banking: The Digital Stamping Journey for Loan Automation
In an era where time is of the essence and convenience reigns supreme, the traditional world of banking has been undergoing a digital makeover.

Reducing Administrative Overhead: How Online Stamp Duty Payments Boost Business Productivity
In the world of modern business, where digital transformation is reshaping operations, it’s essential to reevaluate conventional processes.

Verifying E-Stamp Certificates Online: A Comprehensive Guide
In an increasingly digital world, the use of electronic documents and certificates is becoming more prevalent.

Transforming Banking: The Digital Lending Revolution and Ensuring Compliance
In today’s rapidly evolving financial landscape, traditional banking practices are being revolutionized by digital innovation.

“Unlocking Open Banking: FAQs, Benefits, Challenges, and Digital KYC Insights”
In the rapidly evolving world of financial services, open banking has emerged as a game-changer.

Effortless Affidavit Creation & E-Stamping Guide | MSB Docs for Seamless Legal Documents
In the world of legal documentation, affidavits are essential tools that serve as sworn statements made under oath.

Data Integrity: Harness Its Power to Seamlessly Transition to Pharma 4.0
In the current digital age, data integrity has become increasingly important as organizations strive to transition into a more automated and efficient

Unlock Compliance: Uncover GAMP 5 for GxP Compliant Computer Systems
GAMP 5 stands for Good Automated Manufacturing Practice (GAMP) 5, an important concept in the GMP industries.

Unlock the Benefits of FDA ISO 13485 and 21 CFR Part 820 Harmonization
The U.S. Food and Drug Administration (FDA) is the governing body that regulates medical device manufacturers.

Unlock Quality Improvement In Pharma W/ Smart Goals
A smart goal is an acronym for Specific, Measurable, Achievable, Relevant and Time-bound. It is an effective method used by many organisations to ensu

Unlock Your Auditing Career: 7 Essential Skills for GMP Internal Auditors
Internal auditing plays an important role in Good Manufacturing Practices (GMP). GMP Internal Auditors must have certain essential skills to perform t

Uncover the UDI System: Why it Matters
The UDI System, short for Unique Device Identification System, is a system used to identify medical devices through the use of labels, barcodes, or ot

Solving Sustainability Challenges: Pharma Industry and Sustainability by Design
The pharmaceutical industry plays a critical role in the health and wellbeing of millions of people all over the world.
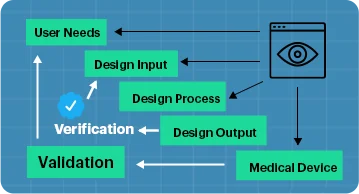
Gain Insight: Design Verification & Validation for Medical Devices
Design verification and design validation are processes used in the production of medical devices.

Risk Assessment: 5 Essential Tools Used by Life Science Companies
Risk assessment is a critical component of life sciences operations, as it helps organizations identify and address potential threats to safety, compl

Life Sciences Tips to Protect from Cyberthreats: Introducing Best Practices
The life sciences sector is at a unique point in its evolution where digital transformation has opened up new opportunities for the industry, while it

Unlock the Keys to Good Manufacturing Practices: A Guide
GMP stands for Good Manufacturing Practices and it is a rigorous set of regulations that must be followed by companies who are involved in the product

Overcome Critical Challenges with QbD: How to Implement QbD Successfully
Quality by Design (QbD) is an approach to drug development and manufacturing that focuses on optimizing the quality of the final product by understand

Automate for Efficiency: Digitize Your Manufacturing with Human-Centric Focus
Human-centric digitization is a way of making certain processes more efficient and efficient at the same time, by incorporating technology.

Digital Revolution: Transform Contract Manufacturing with Digital Technologies
Digital transformation has revolutionized the contract manufacturing industry, allowing companies to streamline operations while increasing efficiency

Navigate CSV Traffic Jams with Validation 4.0: How to Automate Compliant Document Delivery
Validation 4.0 is a new system designed to help teams deliver documents and content quickly while still maintaining regulatory compliance.
Deliver Results with Location Flexibility: Improve Talent Acquisition & Retention
Location flexibility is a crucial element in talent acquisition and retention. It helps to provide job seekers with the freedom they need to work when

Master M&A integration: 7 Steps for Integrating Inherited Talent
Mergers and acquisitions (M&A) often occur due to the need to bring together two or more entities in order to create a larger, more successful or
Enterprise Organizations: Discover How to Improve Your Payroll Processes
Payroll processes are essential to the success of any enterprise organization. These processes ensure that employees are compensated accurately and on

Stand Out from Uncertainty: A Hiring Strategy Guide for Enterprise Businesses
The current global economic climate has created a lot of uncertainty. Businesses of all sizes are feeling the impact of sudden shifts in the job marke

Understand EOR-Sponsored Visas: A Guide for Enterprise Businesses
EOR-Sponsored Visas are a program offered by the US government that allows foreign nationals to work in the US for up to six years.

Get the US Payroll Tax Guide: Alaska to Master Withholding & Filing
The world of payroll taxes can be complicated and knowing the laws and regulations for each state is essential to ensuring that your business is compl

Don’t miss out: Get the Lowdown on US Payroll Tax in Wyoming
Payroll taxes are taxes that are taken from an employee’s salary before they receive their remaining wages. This money is then paid to the state and

Discover the True Cost of Hiring Employees: From Recruitment to Onboarding
Recruiting and onboarding a new employee is often seen as a costly investment for businesses, but the true cost of hiring isn’t always clear.
Avoid Costly Mistakes: Five Common Payroll Blunders
Payroll mistakes can be costly for businesses, both financially and in terms of company reputation.

Unlock the Performance Puzzle: How to Craft a Pay-for-Performance Strategy
A pay-for-performance strategy is a way for organizations to reward their employees for meeting and exceeding goals and objectives.
Secure Your ChatGPT & AI Tech – Uncover Security Threats & Mitigate Risks
ChatGPT is an AI chatbot technology developed by OpenAI, a San Francisco-based company. It uses natural language processing to generate human-like res

Unlocking Smarter Agreements: How AI is Transforming Contracts
Artificial Intelligence (AI) has revolutionized the way businesses work. It can boost efficiency through automation, reduce costs, and improve custome

Asia-Pacific: Navigating HR Transformation in 2023
The Asia-Pacific region is undergoing rapid changes in terms of workforce landscape and human resources strategies.

Uncover the Fundamentals: A CISOs Guide to FedRAMP
FedRAMP is an acronym for the Federal Risk and Authorization Management Program. Devised under a collaborative effort between the government, industry

Discover the Top Compliance Challenges Facing Fast-Growing Businesses
Compliance is an important issue for all companies, but it can be especially challenging for fast-growing companies.

Discover the Pros & Cons of Pharmaceutical Contract Manufacturing
Pharmaceutical contract manufacturing helps businesses meet the increasingly complex demands of the industry.

Mastering Clinical Trials: Guarantee Quality with 4 Essential Phases
Clinical trials are essential for medical research and have proven to be a key element in advancing medical treatments and developing new drugs.
Unlock Pharmaceutical QA Mastery with 4 Easy Steps!
Today, the world of pharmaceuticals is becoming increasingly complex. With global health concerns rising to the forefront, pharmaceutical companies ar
Step-by-Step Guide to Starting a Pharmaceutical Company
Today, the world of pharmaceuticals is becoming increasingly complex. With global health concerns rising to the forefront, pharmaceutical companies ar

Unlock the Secrets of cGMP: 7 Experts Explain
Good Manufacturing Practice (GMP) is a set of guidelines, established by the World Health Organization (WHO), that focus on pharmaceutical manufacturi
Unpacking the Differences: ISO 9001 vs ISO 13485 for Medical Devices
ISO 9000 is an international quality management system (QMS) standard, and ISO 13485 is a QMS Standard specifically designed for medical devices.

Reduce Risk & Reap Benefits: Everything You Need to Know About Design Controls for Med Devices
Design Controls are an essential part of designing and developing any medical device. They help to ensure the safety, effectiveness and quality of a p

Dive Deeper Into Risk Management for Medical Device Startups
Risk management is an essential part of any business, and medical device startups are no exception. Without effective risk management, medical device

Unlock the Secrets of 21 CFR Part 11 Compliance for Medical Device Mfgs
21 CFR Part 11 is a set of regulations developed by the United States Food and Drug Administration (FDA) to enhance the protection of electronic recor

Master FDA 21 CFR 820 Compliance: The 6 Biggest Risk Areas in 2023
The regulation and compliance of FDA 21 CFR 820 is an important element for any organization or company that produces, markets, stores, distributes, o

Pass Your ISO 13485 Audit: All You Need to Know!
ISO 13485 is a global quality management system (QMS) standard used by medical device manufacturers.

Top 4 Reasons Behind Failure of Patient Engagement Solutions in Healthcare
As one of the crucial stakeholders of health care, patients are involved in various aspects of treatment, leading to various controversial opinions ab

10 Surprising Stats about eSignatures
The world of e-signatures reaches far beyond signing. To put it into perspective, here are ten interesting statistics that might surprise you.

Streamlining Healthcare Operations: The Power of Electronic Signatures
For years, healthcare organizations have used wet signatures to establish consent. Lately, document sign-offs via PDFs have become more popular.

What is the difference Between a Digital Signature and an Electronic Signature
It’s common for teams to use the words digital signature and electronic signature interchangeably. In reality, digital signatures and
Wet Signature vs Electronic Signature What Are Wet Signatures & When Are They Required
A wet signature is a sign made with a pen or other writing device on a hard copy in business and law. A wet signature is signing a

Shortcomings Of Patient Engagement Journeys & How Digitalization Is Changing the Game
Healthcare providers such as Medicare Service Providers (MSPs) have many priorities, including initial enrollment or intake of new patients,

Electronic signatures– How Document Digitization is Disrupting the Automotive Industry
One of the biggest headlines in the industry is that Fortune Business Insights believes the global Digital Signature Market will reach USD 35.03

Falling Behind These 5 CIO Priorities Will Cost Your Business to Lose 60-90% of Your Revenue
The economic effects of the coronavirus are becoming evident as it spreads over the world. The pandemic has caused significant corporate disrupti

21 CFR Part 11 eSignature Compliance Checklist Here’s What You Need to Know About FDA Regulations (Updated)
Keeping sensitive data and private information secure is extremely important as well as complex

21 CFR Part 11 eSignature Compliance Checklist Here’s What You Need to Know About FDA Regulations (Updated)
Keeping sensitive data and private information secure is extremely important as well as complex

21 CFR Part 11 Compliance Checklist to Follow
21 CFR Part 11 outlines the FDA’s requirements for the integrity, quality, and compliance of digital documents and also signatures.
Top 10 Enterprise Electronic Signature Software to Improve Organizational Efficiency in 2023
Do you have internal applications that you need to sign off for your business? Procuring signed documents can be a tricky business.

6 Indicators That Can Make or Break Your Document Workflow System
An average office-goer experiences 10,000 sheets of paper every year. Papers, printers, tools, paperclips, folders, staplers, and so on

5 Best Electronic Signature Vendors of 2023
When keeping a physical distance is of top concern, companies can efficiently execute their processes using Electronic Signature capabilities.

Top 5 Electronic Signature APIs that can Replace DocuSign API
Everything is going through a digital transformation nowadays, including how we sign documents. Digital signatures, also known as electronic sig

What’s In It For Businesses Building vs Buying an eSignature API
Whether you’re a small business owner, a consultant, a specialist, or a huge corporation

Which is the top enterprise eSignature solution
Do you have internal applications that you need to sign off for your business? Procuring signed documents can be a tricky business.

MSBs-3s-motto-save-paper-save-time-and-save-resources
Savings in all forms is extremely desirable by all businesses operating across the globe today. These could be money savings, time savings or savings

Digital Documentation “has saved millions” for these organizations, why not you?
Time is money, and money saved is money earned! Well, we all agree with this, and so do those zillions of businesses spread across the globe, who have

Digital Documentation – A Surefire method for Indian Organizations to avoid tedious paperwork
Organizations and businesses in India are rapidly advancing, innovating every now and then and making good use of technology to streamline thei

Digitizing Onboarding experience for HR professionals and New Hires
Attracting and retaining top talent is the most challenging task these days.

Cure Patient Signature Headaches with Improved Digital Healthcare
In 2019, digitization is dominating every market across the board. Yet the digital nature of eSignature technology remains an obstacle to many healthc

21 CFR Part 11 and Good Documentation Practices in Pharmaceutical Industries
It has been said that in the pharmaceutical industry, “If it isn’t documented, it didn’t happen.

Simplify Your Worklife by Going Digital!
Digital India is a visionary initiative set forth by the Indian government to transform India by leveraging the power of information technology. As a
Here’s How Aadhaar eSign is Changing Signing for the Better
Aadhaar e-KYC (Know Your Customer) has left a noticeable print on India. The Aadhaar eSign is a unique, electronic identifier.

Debunking Common eSignature Myths
eSignatures are a growing staple of mainstream technology. As with any new innovation, doubts and myths arise with increasing popularity. With misinfo

Don’t settle for a one-size-fits-all signature solution.
Not all industries are the same. Your business is different than other businesses; so, you need a stress-free solution that can be customized to all y

Going Green with eSignatures
Here at MSB going paperless is about more than saving time and money. It’s also about minimizing our impact on the environment. That’s why we’re
Automating Pharma and Patient Healthcare
In the medical world, companies cannot afford to waste time and money on tedious manual processes such as faxing or mailing overnight documents. Also,

A road to Digital Success in Banks
The eSignature market across banking, insurance, and commerce has evolved. Studies show that at least 15% of revenue is spent

Filing Taxes Can be a Nightmare. With MSB, It Doesn’t Have to Be.
Many of us shudder at the thought of tax season. We don’t look forward to navigating heaps of paperwork and forms. These processes can be overly com

Bridging the Paper Gap in Human Resources
Organizations of all sizes are moving to electronic signatures to transform their HR processes. Looking at the results, it’s no wonder why. eSignatu

Instant Insurance via MSB
When consumers are offered the option to either eSign or sign the traditional way with pen and paper, they overwhelmingly prefer to eSign—a fact tha
Questions you should ask yourself before selecting an e-signature solution
Adopting and implementing electronic signatures to increase the efficiency and profitability of your business is a significant step. Implementing eSig

Is Paper Damaging Your Vendor Relationships? MSB Can Help.
Maintaining a healthy relationship with vendors is of vital importance to companies. Today all small and big enterprises are moving from paper-driven

MSB Makes Excellent Customer Service Effortless
What makes a business successful? Organizations that provide consistent, legendary service to their customers achieve higher satisfaction levels and i

The Power of Smart Paperless Transactions
Did you know 15% of organizational revenue is spent on creating, managing, and distributing documents? Or that 60% of employee time is spent working w
Document Automation – Future of Business Process Operations
There are millions of businesses that operate across the globe, dealing with different types of products and services, catering to different sections

Satisfy Global Compliance and Data Security Needs with Digital Signatures
Business leaders are well aware of the need to protect themselves and their company with legally binding contracts. An organization’s reputation a

Step Up Your Sales Game with MSB
Serious costs and risks plague manual, paper-based document transactions. Paper is fragile and destructible; it can be easily misplaced and damaged. U

Digital Healthcare: Transforming the Patient Experience
Hospitals that continue to rely on manual, paper-based processes know how much paper plagues patients. These outdated methods hinder hospitals’ abil

MSB’s TeamRoom has a Room for All
No matter if you are running a business at a large or small scale, collaboration is one of the essential things that can easily make or break your bus

Time to Get Out of PSS (Print, Sign, Scan) Routine
The rapid increase in technological advancements has a substantial impact on many businesses. With this, MSB Docs is always trying to introduce the be

Digital Onboarding with MSB Docs – A Faster, Easier & More Transparent Process
The beginning of a new employee’s journey in an organization always begins with the onboarding process. Smoother, the onboarding is carried out smoo

Healthcare Data Leak – A Repeated Warning for Healthcare Providers
n the latest healthcare data leak, the German firm, Greenbone Networks has reported that over a million medical records and 121 Million medical images
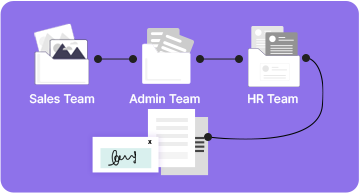
Which Departments Need Digital Transformation with MSB Docs?
Drafting, printing, scanning, and filing documents – a process that was said to be a necessary part of any organization is now getting easier and ea

3 Easy Steps to Get Your Sales Contract Signed in Minutes
Salespeople working with any small or large enterprises are spending most of their time in old-school or standard sales practices. In return, these pr
Handle Your Documentation Smartly with MSB Docs OCR
Have you ever jumped between multiple software and applications just to finish a single document? If yes, then you know the struggle very well. Since

Guide for Smooth Transition to Work from Home
According to the World Health Organization, Coronavirus (COVID-19) has officially been categorized as a pandemic. That means it has spread across a va

Boost Business Productivity with MSB Docs Integrations
Have you ever jumped between multiple software and applications just to finish a single document? If yes, then you know the struggle very well. Since

Work Smartly with Smart Categorization Offered by MSB Docs
The rapid increase in technological advancements has a substantial impact on many businesses. With this, MSB Docs is always trying to introduce the be

5 Myths About Electronic Signatures You Must Stop Believing
Over the years, we have witnessed unprecedented transformation on the technological front, which has probably doubled these days due to COVID-19 crisi

Does MSB Docs Conform with HIPAA Compliance?
Ink and paper are losing their momentum in many industries as almost every industry is trying to transform its processes digitally.

Electronic Contracts Vs Traditional Contracts – Let’s bridge the gap!
With the increase in new avenues of digital transformation, the need for paperless processes has become even more significant. Instead of creating, pr

Gxp Compliance and Validation: How Going Paperless Saves Your Efforts
If you are running a business in life sciences or other regulated industries, you must be knowing what intensifying competition is. The shrinking marg

MSB Docs Digital Onboarding – Guide to Eliminate Paper from Paperwork
The onboarding process involves a lot of documentation to be done, which requires in-person signing. With traditional practices, this turns out to be
How Information Security is Ingrained in Our People, Processes & Technologies
MSB Docs is the smart document solution software product that treats information security as a part of its DNA. All the information assets

3 Ways We Cut-Short the MSB Docs User Acceptance Testing
Every software is designed and developed for the ease and usability of end-users. When these criteria are missed or avoided by the team, then, the fin

How Did MSB Docs Become a Lifesaver for Real Estate Business
Real Estate is dynamically growing at high speed in this business world. With this, the competition is getting tough each passing day. Since closing r

4 Ways Paperwork Burns a Hole in Your Pocket
Today, even after two decades of digitization, paper is an active part of business processes. From memos to contracts; sales invoices to payslips
Digital Workflows – A One-stop Solution for Distributed Workforce
The first step is always the hardest, but it is a must to unleash the opportunities waiting ahead.

Bid Adieu to Invoice Delays & Say Hello to Astute Transactions
Since we are living in the era of “one-click order and same-day delivery,” invoice clearance becomes quintessential. But if you stubbornly hold
How Going Digital Fast Tracks Your Salesforce Automated Process
No matter if a business is running at a small, medium, or large scale, improved productivity remains the most important KPI

Is your Life Sciences Business Ready to Face Coronavirus Disruptions?
The coronavirus pandemic is likely to bring a challenging situation for the business world. Most of the business associations have already started wor
Selecting a Smart Document Solution? 5 Facts Before you Do
Managing documents can be a tedious task – and this amplifies with “paper” based documentation. So smart decision-makers like

Go Contactless with eSignatures using MSB Docs Smart Document Solution!
We are in the midst of unusual times led by Covid-19, where businesses are mostly operating remotely and human interactions are being fairly minimized

UETA – Here’s What You Need to Know Before Going Digital
If you have stepped into the world of electronic signatures, you might have come across the acronym UETA a lot. This blog post talks about UETA and ho

How Digital signatures Work?
Digital signatures, a type of electronic signatures/ e-signatures, are like electronic fingerprints. Unique to every person, like our pen-paper signat

Online Document Security & Privacy: Does it matter?
Almost every business sector has been affected by cyber-attacks in one or the other way. Especially IT, BFSI and healthcare sectors suffered

How Digital Onboarding Elevates Customer Experience?
You never get a second chance to make a good first impression

How Does MSB Docs Help Your Auto Shop Go Paperless?
Whether you are digital-savvy or not, digitizing paper-based business processes has become a new norm.
How MSB Docs Covered the COVID-19 Disruptions in Life Sciences Industry?
With the global industry disruption, COVID-19 affected the life sciences industry the most. Despite tracking external news events and getting early wa

Tips and Suggestions on Going Paperless in Your Office
Creating a paperless office was a lofty dream, a thing of the future, even a few years ago. Thanks to the rapid advent of technology,

Sign IEP Forms Remotely with MSB Docs
Schools are the most affected area of the COVID-19 pandemic! Although teaching found a way out by switching to online classes,

Migrating to MSB Docs for 100% Reliability & Higher Usability
In this digital age, there are plenty of document transaction solutions that are designed to deliver game-changing results.

Why MSB Docs Web Forms are Ideal for Enterprises?
In today’s fast-growing business world, almost every enterprise needs to generate on-demand, self-service documents for signatures.

21 CFR Part 11 Compliance Checklist
Every life sciences company that either sells or proposes to sell any kind of medical device has to follow the requirements under

5 Ways Legal Contract Management Can Help In Automation
Manual, resource-draining tasks have been dominating legal departments and the legal industry itself.

Top 9 Digital Transformation Trends in Pharmaceutical Industry (Update 2021)
The pharmaceutical industry has seen massive growth in recent years, and it clearly shows the upward trend for 2021 as well. Technological advances ar

Why Qualified Electronic Signatures are the Most Secure Type of eSignatures?
Electronic Identification, Authentication, and Trust Services (eIDAS) is the EU regulation on electronic identification and trust services for electro

How Electronic Signature Can Help Banks To Move Ahead
Electronic signatures are taking the global market by storm. In 2019 the global digital signature market accounted for $1,534.8 million.

5 Ways to Protect Sensitive Healthcare Data
Data security remains a key issue in almost every industry. The reason being companies don’t understand that data security goes beyond compliance.

Digital Signature – A Complete Guide
A signature holds the power to validate a document’s integrity and authenticity. A digital signature’s work is kind of similar but it uses a mathe

What is Contract Management? & Why it’s important?
The term Contract Management denotes a process related to business. This business process includes contract formation, contract evaluation

7 important digital signature uses in 2023
A digital signature is precisely an electronic form of signature used to authenticate any digital document and identity of the signer.

Why pharmaceutical industry loses Billions of dollars every year?
Pharma industry is one of the largest industries that is totally built on innovation, involving a huge investment in research and development (R&
Create your Online Signature with MSB Docs Now
A signed document indicates its authenticity and proves that it is valid. Today in the modern world where technology is growing at a fast pace,

Top eSignature use cases for small business
The manual process of signing a document is without a doubt timid and time-consuming. It is a hassle for business.
How MSB Docs CRA Scan App Helps in Good Clinical Practice (GCP)
Advancement in the prevention, medication, and treatment of healthcare problems has only been possible because of the good clinical practices which

Automate for Efficiency: Digitize Your Manufacturing with Human-Centric Focus
Human-centric digitization is a way of making certain processes more efficient and efficient at the same time, by incorporating technology.

Top 4 Reasons Behind Failure of Patient Engagement Solutions in Healthcare
As one of the crucial stakeholders of health care, patients are involved in various aspects of treatment, leading to various controversial opinions ab

Streamlining Healthcare Operations: The Power of Electronic Signatures
For years, healthcare organizations have used wet signatures to establish consent. Lately, document sign-offs via PDFs have become more popular.

Shortcomings Of Patient Engagement Journeys & How Digitalization Is Changing the Game
Healthcare providers such as Medicare Service Providers (MSPs) have many priorities, including initial enrollment or intake of new patients,

Falling Behind These 5 CIO Priorities Will Cost Your Business to Lose 60-90% of Your Revenue
The economic effects of the coronavirus are becoming evident as it spreads over the world. The pandemic has caused significant corporate disrupti

MSBs-3s-motto-save-paper-save-time-and-save-resources
Savings in all forms is extremely desirable by all businesses operating across the globe today. These could be money savings, time savings or savings

Digital Documentation “has saved millions” for these organizations, why not you?
Time is money, and money saved is money earned! Well, we all agree with this, and so do those zillions of businesses spread across the globe, who have

Digital Documentation – A Surefire method for Indian Organizations to avoid tedious paperwork
Organizations and businesses in India are rapidly advancing, innovating every now and then and making good use of technology to streamline thei

Digitizing Onboarding experience for HR professionals and New Hires
Attracting and retaining top talent is the most challenging task these days.

Cure Patient Signature Headaches with Improved Digital Healthcare
In 2019, digitization is dominating every market across the board. Yet the digital nature of eSignature technology remains an obstacle to many healthc

Simplify Your Worklife by Going Digital!
Digital India is a visionary initiative set forth by the Indian government to transform India by leveraging the power of information technology. As a

Here’s How Aadhaar eSign is Changing Signing for the Better
Aadhaar e-KYC (Know Your Customer) has left a noticeable print on India. The Aadhaar eSign is a unique, electronic identifier.

Debunking Common eSignature Myths
eSignatures are a growing staple of mainstream technology. As with any new innovation, doubts and myths arise with increasing popularity. With misinfo

Don’t settle for a one-size-fits-all signature solution.
Not all industries are the same. Your business is different than other businesses; so, you need a stress-free solution that can be customized to all y

Going Green with eSignatures
Here at MSB going paperless is about more than saving time and money. It’s also about minimizing our impact on the environment. That’s why we’re

Automating Pharma and Patient Healthcare
In the medical world, companies cannot afford to waste time and money on tedious manual processes such as faxing or mailing overnight documents. Also,

Filing Taxes Can be a Nightmare. With MSB, It Doesn’t Have to Be.
Many of us shudder at the thought of tax season. We don’t look forward to navigating heaps of paperwork and forms. These processes can be overly com

Bridging the Paper Gap in Human Resources
Organizations of all sizes are moving to electronic signatures to transform their HR processes. Looking at the results, it’s no wonder why. eSignatu

Questions you should ask yourself before selecting an e-signature solution
Adopting and implementing electronic signatures to increase the efficiency and profitability of your business is a significant step. Implementing eSig

Is Paper Damaging Your Vendor Relationships? MSB Can Help.
Maintaining a healthy relationship with vendors is of vital importance to companies. Today all small and big enterprises are moving from paper-driven

MSB Makes Excellent Customer Service Effortless
What makes a business successful? Organizations that provide consistent, legendary service to their customers achieve higher satisfaction levels and i

The Power of Smart Paperless Transactions
Did you know 15% of organizational revenue is spent on creating, managing, and distributing documents? Or that 60% of employee time is spent working w

Document Automation – Future of Business Process Operations
There are millions of businesses that operate across the globe, dealing with different types of products and services, catering to different sections

Satisfy Global Compliance and Data Security Needs with Digital Signatures
Business leaders are well aware of the need to protect themselves and their company with legally binding contracts. An organization’s reputation a

Step Up Your Sales Game with MSB
Serious costs and risks plague manual, paper-based document transactions. Paper is fragile and destructible; it can be easily misplaced and damaged. U

Digital Healthcare: Transforming the Patient Experience
Hospitals that continue to rely on manual, paper-based processes know how much paper plagues patients. These outdated methods hinder hospitals’ abil

MSB’s TeamRoom has a Room for All
No matter if you are running a business at a large or small scale, collaboration is one of the essential things that can easily make or break your bus

Time to Get Out of PSS (Print, Sign, Scan) Routine
The rapid increase in technological advancements has a substantial impact on many businesses. With this, MSB Docs is always trying to introduce the be

Digital Onboarding with MSB Docs – A Faster, Easier & More Transparent Process
The beginning of a new employee’s journey in an organization always begins with the onboarding process. Smoother, the onboarding is carried out smoo

Healthcare Data Leak – A Repeated Warning for Healthcare Providers
n the latest healthcare data leak, the German firm, Greenbone Networks has reported that over a million medical records and 121 Million medical images

Which Departments Need Digital Transformation with MSB Docs?
Drafting, printing, scanning, and filing documents – a process that was said to be a necessary part of any organization is now getting easier and ea

3 Easy Steps to Get Your Sales Contract Signed in Minutes
Salespeople working with any small or large enterprises are spending most of their time in old-school or standard sales practices. In return, these pr

Handle Your Documentation Smartly with MSB Docs OCR
Have you ever jumped between multiple software and applications just to finish a single document? If yes, then you know the struggle very well. Since

Guide for Smooth Transition to Work from Home
According to the World Health Organization, Coronavirus (COVID-19) has officially been categorized as a pandemic. That means it has spread across a va

Boost Business Productivity with MSB Docs Integrations
Have you ever jumped between multiple software and applications just to finish a single document? If yes, then you know the struggle very well. Since

Work Smartly with Smart Categorization Offered by MSB Docs
The rapid increase in technological advancements has a substantial impact on many businesses. With this, MSB Docs is always trying to introduce the be

Electronic Contracts Vs Traditional Contracts – Let’s bridge the gap!
With the increase in new avenues of digital transformation, the need for paperless processes has become even more significant. Instead of creating, pr

Gxp Compliance and Validation: How Going Paperless Saves Your Efforts
If you are running a business in life sciences or other regulated industries, you must be knowing what intensifying competition is. The shrinking marg

How Information Security is Ingrained in Our People, Processes & Technologies
MSB Docs is the smart document solution software product that treats information security as a part of its DNA. All the information assets

3 Ways We Cut-Short the MSB Docs User Acceptance Testing
Every software is designed and developed for the ease and usability of end-users. When these criteria are missed or avoided by the team, then, the fin

How Did MSB Docs Become a Lifesaver for Real Estate Business
Real Estate is dynamically growing at high speed in this business world. With this, the competition is getting tough each passing day. Since closing r

4 Ways Paperwork Burns a Hole in Your Pocket
Today, even after two decades of digitization, paper is an active part of business processes. From memos to contracts; sales invoices to payslips

Digital Workflows – A One-stop Solution for Distributed Workforce
The first step is always the hardest, but it is a must to unleash the opportunities waiting ahead.

Bid Adieu to Invoice Delays & Say Hello to Astute Transactions
Since we are living in the era of “one-click order and same-day delivery,” invoice clearance becomes quintessential. But if you stubbornly hold

How Going Digital Fast Tracks Your Salesforce Automated Process
No matter if a business is running at a small, medium, or large scale, improved productivity remains the most important KPI

Is your Life Sciences Business Ready to Face Coronavirus Disruptions?
The coronavirus pandemic is likely to bring a challenging situation for the business world. Most of the business associations have already started wor

Selecting a Smart Document Solution? 5 Facts Before you Do
Managing documents can be a tedious task – and this amplifies with “paper” based documentation. So smart decision-makers like

Go Contactless with eSignatures using MSB Docs Smart Document Solution!
We are in the midst of unusual times led by Covid-19, where businesses are mostly operating remotely and human interactions are being fairly minimized

UETA – Here’s What You Need to Know Before Going Digital
If you have stepped into the world of electronic signatures, you might have come across the acronym UETA a lot. This blog post talks about UETA and ho

How Digital signatures Work?
Digital signatures, a type of electronic signatures/ e-signatures, are like electronic fingerprints. Unique to every person, like our pen-paper signat

Online Document Security & Privacy: Does it matter?
Almost every business sector has been affected by cyber-attacks in one or the other way. Especially IT, BFSI and healthcare sectors suffered

How Digital Onboarding Elevates Customer Experience?
You never get a second chance to make a good first impression

How Does MSB Docs Help Your Auto Shop Go Paperless?
Whether you are digital-savvy or not, digitizing paper-based business processes has become a new norm.

How MSB Docs Covered the COVID-19 Disruptions in Life Sciences Industry?
With the global industry disruption, COVID-19 affected the life sciences industry the most. Despite tracking external news events and getting early wa

Tips and Suggestions on Going Paperless in Your Office
Creating a paperless office was a lofty dream, a thing of the future, even a few years ago. Thanks to the rapid advent of technology,

Migrating to MSB Docs for 100% Reliability & Higher Usability
In this digital age, there are plenty of document transaction solutions that are designed to deliver game-changing results.

Why MSB Docs Web Forms are Ideal for Enterprises?
In today’s fast-growing business world, almost every enterprise needs to generate on-demand, self-service documents for signatures.

5 Ways Legal Contract Management Can Help In Automation
Manual, resource-draining tasks have been dominating legal departments and the legal industry itself.

Top 9 Digital Transformation Trends in Pharmaceutical Industry (Update 2021)
The pharmaceutical industry has seen massive growth in recent years, and it clearly shows the upward trend for 2021 as well. Technological advances ar

5 Ways to Protect Sensitive Healthcare Data
Data security remains a key issue in almost every industry. The reason being companies don’t understand that data security goes beyond compliance.

Digital Signature – A Complete Guide
A signature holds the power to validate a document’s integrity and authenticity. A digital signature’s work is kind of similar but it uses a mathe

What is Contract Management? & Why it’s important?
The term Contract Management denotes a process related to business. This business process includes contract formation, contract evaluation

7 important digital signature uses in 2023
A digital signature is precisely an electronic form of signature used to authenticate any digital document and identity of the signer.

Why pharmaceutical industry loses Billions of dollars every year?
Pharma industry is one of the largest industries that is totally built on innovation, involving a huge investment in research and development (R&

Create your Online Signature with MSB Docs Now
A signed document indicates its authenticity and proves that it is valid. Today in the modern world where technology is growing at a fast pace,

Top eSignature use cases for small business
The manual process of signing a document is without a doubt timid and time-consuming. It is a hassle for business.

How MSB Docs CRA Scan App Helps in Good Clinical Practice (GCP)
Advancement in the prevention, medication, and treatment of healthcare problems has only been possible because of the good clinical practices which

Revolutionizing FMCG Vendor Onboarding with MSB Docs eSignature: Streamlined Efficiency
The Fast-Moving Consumer Goods (FMCG) industry is a highly competitive and dynamic sector that relies on efficient supply chain management.

“Unlocking Efficiency and Security: The Benefits of eKYC for Customer Onboarding”
In today’s fast-paced digital world, customer onboarding is a critical process for businesses across various industries.

Deliver Results with Location Flexibility: Improve Talent Acquisition & Retention
Location flexibility is a crucial element in talent acquisition and retention. It helps to provide job seekers with the freedom they need to work when

Master M&A integration: 7 Steps for Integrating Inherited Talent
Mergers and acquisitions (M&A) often occur due to the need to bring together two or more entities in order to create a larger, more successful or

Enterprise Organizations: Discover How to Improve Your Payroll Processes
Payroll processes are essential to the success of any enterprise organization. These processes ensure that employees are compensated accurately and on

Stand Out from Uncertainty: A Hiring Strategy Guide for Enterprise Businesses
The current global economic climate has created a lot of uncertainty. Businesses of all sizes are feeling the impact of sudden shifts in the job marke

Discover the True Cost of Hiring Employees: From Recruitment to Onboarding
Recruiting and onboarding a new employee is often seen as a costly investment for businesses, but the true cost of hiring isn’t always clear.

Avoid Costly Mistakes: Five Common Payroll Blunders
Payroll mistakes can be costly for businesses, both financially and in terms of company reputation.

Unlock the Performance Puzzle: How to Craft a Pay-for-Performance Strategy
A pay-for-performance strategy is a way for organizations to reward their employees for meeting and exceeding goals and objectives.

Asia-Pacific: Navigating HR Transformation in 2023
The Asia-Pacific region is undergoing rapid changes in terms of workforce landscape and human resources strategies.

RBI Revamps Digital Lending: DLG Permitted to Streamline Loans
With more and more of our lives transitioning to the digital space, financial services are no exception. In an increasingly globalised and data-driven

Revolutionizing Banking: The Digital Stamping Journey for Loan Automation
In an era where time is of the essence and convenience reigns supreme, the traditional world of banking has been undergoing a digital makeover.

Transforming Banking: The Digital Lending Revolution and Ensuring Compliance
In today’s rapidly evolving financial landscape, traditional banking practices are being revolutionized by digital innovation.

Don’t miss out: Get the Lowdown on US Payroll Tax in Wyoming
Payroll taxes are taxes that are taken from an employee’s salary before they receive their remaining wages. This money is then paid to the state and

A road to Digital Success in Banks
The eSignature market across banking, insurance, and commerce has evolved. Studies show that at least 15% of revenue is spent

Instant Insurance via MSB
When consumers are offered the option to either eSign or sign the traditional way with pen and paper, they overwhelmingly prefer to eSign—a fact tha

5 Myths About Electronic Signatures You Must Stop Believing
Over the years, we have witnessed unprecedented transformation on the technological front, which has probably doubled these days due to COVID-19 crisi

Does MSB Docs Conform with HIPAA Compliance?
Ink and paper are losing their momentum in many industries as almost every industry is trying to transform its processes digitally.

MSB Docs Digital Onboarding – Guide to Eliminate Paper from Paperwork
The onboarding process involves a lot of documentation to be done, which requires in-person signing. With traditional practices, this turns out to be

Get the Lowdown on Affidavits + their E-Stamping Requirements
Affidavits are important documents that are sworn in writing and may be used as legal evidence in court.

Unlocking Efficiency: How Digital Stamping Transforms Loan Disbursement for NBFCs
In the rapidly evolving landscape of financial services, Non-Banking Financial Companies (NBFCs) are playing a pivotal role in providing loans

Transforming Business: 5 Critical Industries Embracing Digital Stamp Solutions
In our rapidly evolving digital landscape, the importance of secure and efficient document authentication cannot be overstated.

E-Stamping: Your Complete Guide to Online Document Authentication
In today’s fast-paced digital world, the way we handle documents and transactions is constantly evolving.

Demystifying Differential Stamp Duty in India: Your Comprehensive Guide
Stamp duty is an essential part of the financial landscape in India, serving as a significant source of revenue for state governments.

Digital Stamping 101: How Banks Are Revolutionizing Document Workflow
In today’s fast-paced digital age, the banking industry is continually evolving to meet the needs of both customers and regulatory authorities.

Digital Stamping Revolution: Efficiency, Security, and Benefits for Businesses
In the digital age, businesses are constantly seeking innovative solutions to streamline their operations and improve efficiency.

Mastering Online Stamp Duty Payment: A 5-Step Guide for Effortless Transactions
Stamp duty is a crucial part of various financial transactions, whether it’s purchasing property, transferring shares, or executing legal d

E-Stamping in India 2023: A Comprehensive Guide to Digital Stamp Duty Payment
E-Stamping, short for electronic stamping, is a digital method of paying stamp duty for various legal documents and transactions.

Reducing Administrative Overhead: How Online Stamp Duty Payments Boost Business Productivity
In the world of modern business, where digital transformation is reshaping operations, it’s essential to reevaluate conventional processes.

Verifying E-Stamp Certificates Online: A Comprehensive Guide
In an increasingly digital world, the use of electronic documents and certificates is becoming more prevalent.

Effortless Affidavit Creation & E-Stamping Guide | MSB Docs for Seamless Legal Documents
In the world of legal documentation, affidavits are essential tools that serve as sworn statements made under oath.

Selecting the Perfect Aadhaar eSignature Vendor: A Comprehensive Guide
Aadhaar-based eSignatures have become an integral part of India’s digital landscape, revolutionizing how businesses and individuals authenticate

eSign Audit Trails: Your Digital Signature Safety Net
In today’s digitally-driven world, electronic signatures, commonly known as eSignatures, have transformed the way we sign and exchange documents

eSign Solutions for NGOs: Supercharge Donations & Fundraising
In the world of non-governmental organizations (NGOs), the ability to efficiently manage donations and fundraising is crucial.

Close Deals Faster with eSignatures: A Comprehensive Guide for Sales Teams
In today’s fast-paced business environment, time is money. For sales teams, closing deals quickly can make the difference between success and st

Comprehensive Guide to eSign Key Fact Statements (KFS) with Digital Signatures
In today’s digital age, the way we handle documents has undergone a significant transformation.

Unlocking the Power of Aadhaar eSign API: Your Guide to Effortless eSignatures
In today’s fast-paced digital world, electronic signatures have transitioned from being a luxury to a necessity.

MeiTY IT Act Amendment Empowered Five Document Types
In today’s digital era, electronic signatures (e-signatures) have emerged as a powerful tool, transforming the way we handle official documents.

eSignatures in Real Estate: Embracing Efficiency with the IT Act Amendment
In a rapidly evolving digital age, real estate transactions are no exception to the transformative power of technology.

Mastering Online Signatures: A Comprehensive Guide for Businesses
In today’s digital age, the business world is constantly evolving, and technological advancements are transforming the way we conduct business.

Sign with Confidence: A Global Guide to E-Signature Laws
In today’s digital age, the world has witnessed a profound transformation in the way businesses operate.

How to Sign Contracts Online | MSB Docs
In today’s digital age, almost every aspect of business and personal life has been transformed by technology.

Mastering Online PDF Signatures: A Step-by-Step Guide for Legal, Secure, and Efficient Signing
In today’s digital world, the need to sign documents securely and efficiently is paramount.

Secure Healthcare Documents with HIPAA Compliant E-Signature Solutions | MSB Docs
In today’s digital age, the healthcare industry faces numerous challenges, and one of the most pressing concerns is ensuring the security and in

Unlock Digital Lending: Here’s How Banks Can Help
Digital lending is revolutionizing the way banks do business. It’s enabling banks to offer paperless loans to customers within a matter of minutes a

Get the Basics of an NDA: 15 Questions to Ask Before Signing
The purpose of this guide is to provide readers with an understanding of Non-Disclosure Agreements (NDAs) and the 15 key questions they should ask the

Make an Impression: Unlock Your Unique Signature
A signature is a mark or symbol representing you. It is a unique, personal identifier that is attached to your identity to show ownership or to certif

Get more Secure: Learn Difference of E-Signature & Digital Signature
An electronic signature and a digital signature are two terms that are sometimes used interchangeably, but there is an important difference between th

What’s The Difference? Get the Lowdown on Agreements vs Contracts
Agreements and contracts are essential elements of our everyday lives, playing a crucial role in the way we conduct business and make commitments.

Grasp the Difference: Contract Management vs Contract Lifecycle Management
Contracts are a foundational element of any successful business. As such, it’s essential to understand the differences between contract management

Discover What Contract Lifecycle Management Is & How It Benefits Businesses
Contract Lifecycle Management (CLM) is a process that helps businesses effectively manage, control and organize the different stages of a contract’s

Digital Revolution: Transform Contract Manufacturing with Digital Technologies
Digital transformation has revolutionized the contract manufacturing industry, allowing companies to streamline operations while increasing efficiency

Unlocking Smarter Agreements: How AI is Transforming Contracts
Artificial Intelligence (AI) has revolutionized the way businesses work. It can boost efficiency through automation, reduce costs, and improve custome

10 Surprising Stats about eSignatures
The world of e-signatures reaches far beyond signing. To put it into perspective, here are ten interesting statistics that might surprise you.

What is the difference Between a Digital Signature and an Electronic Signature
It’s common for teams to use the words digital signature and electronic signature interchangeably. In reality, digital signatures and

Wet Signature vs Electronic Signature What Are Wet Signatures & When Are They Required
A wet signature is a sign made with a pen or other writing device on a hard copy in business and law. A wet signature is signing a

Electronic signatures– How Document Digitization is Disrupting the Automotive Industry
One of the biggest headlines in the industry is that Fortune Business Insights believes the global Digital Signature Market will reach USD 35.03

21 CFR Part 11 eSignature Compliance Checklist Here’s What You Need to Know About FDA Regulations (Updated)
Keeping sensitive data and private information secure is extremely important as well as complex

Top 10 Enterprise Electronic Signature Software to Improve Organizational Efficiency in 2023
Do you have internal applications that you need to sign off for your business? Procuring signed documents can be a tricky business.

6 Indicators That Can Make or Break Your Document Workflow System
An average office-goer experiences 10,000 sheets of paper every year. Papers, printers, tools, paperclips, folders, staplers, and so on

5 Best Electronic Signature Vendors of 2023
When keeping a physical distance is of top concern, companies can efficiently execute their processes using Electronic Signature capabilities.

Top 5 Electronic Signature APIs that can Replace DocuSign API
Everything is going through a digital transformation nowadays, including how we sign documents. Digital signatures, also known as electronic sig

What’s In It For Businesses Building vs Buying an eSignature API
Whether you’re a small business owner, a consultant, a specialist, or a huge corporation

Which is the top enterprise eSignature solution
Do you have internal applications that you need to sign off for your business? Procuring signed documents can be a tricky business.

Don’t settle for a one-size-fits-all signature solution.
Not all industries are the same. Your business is different than other businesses; so, you need a stress-free solution that can be customized to all y

Going Green with eSignatures
Here at MSB going paperless is about more than saving time and money. It’s also about minimizing our impact on the environment. That’s why we’re

Go Contactless with eSignatures using MSB Docs Smart Document Solution!
We are in the midst of unusual times led by Covid-19, where businesses are mostly operating remotely and human interactions are being fairly minimized

How Digital signatures Work?
Digital signatures, a type of electronic signatures/ e-signatures, are like electronic fingerprints. Unique to every person, like our pen-paper signat

Sign IEP Forms Remotely with MSB Docs
Schools are the most affected area of the COVID-19 pandemic! Although teaching found a way out by switching to online classes,

21 CFR Part 11 Compliance Checklist
Every life sciences company that either sells or proposes to sell any kind of medical device has to follow the requirements under

Why Qualified Electronic Signatures are the Most Secure Type of eSignatures?
Electronic Identification, Authentication, and Trust Services (eIDAS) is the EU regulation on electronic identification and trust services for electro

How Electronic Signature Can Help Banks To Move Ahead
Electronic signatures are taking the global market by storm. In 2019 the global digital signature market accounted for $1,534.8 million.

Unlocking Healthtech Success:Understanding Business Models
When it comes to the world of healthcare, technology is becoming increasingly essential for medical organizations to increase patient satisfaction, re

Transform Healthcare: Learn How AI & Automation are Changing Medicine
With the introduction of Artificial Intelligence (AI) and automation, the medical information industry is undergoing a radical transformation.

Don’t Miss Out: The Best Medical Events in 2nd Half of ’23
Medical device events are an important part of the larger medical device industry. Attending these events can help you to stay up-to-date with the lat

Aadhaar eKYC Advantages: Speed, Savings, and Security | The Digital Transformation
In the digital age, identity verification is a crucial component of countless services and transactions. Aadhaar eKYC (Electronic Know Your Customer)

Strengthening Online Banking Security: How KYC Fights Identity Theft and Fraud
In today’s digital age, online banking and financial transactions have become an integral part of our lives.

Optimizing Credit Card Onboarding with Video KYC: Streamlining the Process
Credit card onboarding, a crucial process in the financial industry, can often be a source of frustration and complexity for both financial institutio

Revamp Your KYC Process for Business Success: 3 Key Changes to Embrace
In today’s rapidly evolving business landscape, one thing remains constant: the importance of the Know Your Customer (KYC) process.

“Transforming MSME Onboarding: How Video KYC Drives Efficiency and Growth”
Micro, Small, and Medium Enterprises (MSMEs) are the lifeblood of many economies, contributing significantly to employment and economic growth.

7 Fascinating Facts About Video KYC and the Role of MSB Docs eKYC Solution
In today’s fast-paced digital world, businesses are constantly seeking innovative solutions to streamline their operations, enhance security, an

Elevating Retail & FMCG Security: The Power of Vendor KYC Verification
In the ever-evolving landscape of retail and Fast-Moving Consumer Goods (FMCG), the efficient procurement and distribution of products are critical fo

Get Smart on KYC: What it Means & Why It Matters
KYC stands for Know Your Customer. It is a process that businesses use to verify the identity of their customers when they sign up for a product or se

Uncover the Must-Have KYC Checklist for a Compliant Program
Having a compliant Know Your Customer (KYC) program is essential for any business, no matter its size or sector.

VIPV KYC: A Comprehensive Guide to Video In Person Verification
In the ever-evolving realm of finance and digital transactions, ensuring the identity and legitimacy of customers is paramount.

Money Laundering in Insurance: Risks, Regulations, and Rewards | MSB Docs
Money laundering is a global menace that affects various sectors, including the insurance industry.

Critical Assessment: RBI’s cKYC High-Risk Designation & the Path to Video KYC Adoption
In the world of financial services, the Know Your Customer (KYC) process stands as a guardian of integrity, transparency, and regulatory complian

“Unlocking Open Banking: FAQs, Benefits, Challenges, and Digital KYC Insights”
In the rapidly evolving world of financial services, open banking has emerged as a game-changer.

Don’t Make These 5 Mistakes When Preparing an IND Filing Plan
Biotech startups are revolutionizing the medical and healthcare industry. From finding new ways to treat illnesses to developing innovative technologi

Don’t Miss Out: 5 Essential Mistakes to Avoid When Writing a Biotech Startup’s Development Plan
An Investigational New Drug (IND) application is a legal document submitted to the Food and Drug Administration (FDA).

Capture the Benefits: Transitioning from Early-Stage to Late-Stage Biotech
Biotechnology is one of the most transformative forces in the world today. It stands poised to revolutionize our understanding of science, medicine, a

Unlock Science: How to Leverage NLP in the Life Sciences
Natural language processing (NLP) has revolutionized many industries, making complex processes easier and more efficient.

Risk Assessment: 5 Essential Tools Used by Life Science Companies
Risk assessment is a critical component of life sciences operations, as it helps organizations identify and address potential threats to safety, compl

Life Sciences Tips to Protect from Cyberthreats: Introducing Best Practices
The life sciences sector is at a unique point in its evolution where digital transformation has opened up new opportunities for the industry, while it

Streamline Vendor Contracts & Onboarding: Automation with MSB Docs
Vendor contracts and onboarding play pivotal roles in managing business relationships with suppliers and service providers.

Document Automation Reality Check: How MSB Docs eSignature Delivers Results
In today’s digital age, businesses across various industries are constantly seeking ways to streamline their operations and enhance efficiency.

Unlocking Efficiency: eSignatures in Franchise Agreements | MSB Docs
In the ever-evolving landscape of business operations, the traditional processes of handling contracts and agreements are rapidly giving way to digita

How to Write Perfectly for CAPA: An Essential Guide
CAPA (Corrective Action/Preventative Action) processes are essential requirements for many businesses in today’s world.

Solve Investigations Right: 5 Essential Mistakes To Avoid
Are you ready to get started on your investigation but don’t know where to begin? Writing an investigation often comes with its own set of challenge

Understand EOR-Sponsored Visas: A Guide for Enterprise Businesses
EOR-Sponsored Visas are a program offered by the US government that allows foreign nationals to work in the US for up to six years.

Unlocking the Power of Digital Contracts: Legal Validity and Enhanced Security
In the digital age, the business landscape is undergoing a profound transformation.

Guarding Against Identity Theft in Online KYC: Tips and Solutions
In today’s digital age, online video KYC (Know Your Customer) has become a crucial component of identity verification processes for businesses a

Achieving Business Compliance: A Deep Dive into KYC Practices | MSB Docs
In today’s global business landscape, where transactions cross borders and industries seamlessly, the need for robust compliance measures has ne

Achieve Total Quality Mgmt – Complete Guide to ISO 13485 Certification
ISO 13485 certification is an internationally recognized set of standards aimed at ensuring that medical device manufacturers have implemented stringe

Unveiled: A Guide to What is FDA 21 CFR Part 111?
The Food and Drug Administration (FDA) created 21 CFR Part 111 in 2007 for the purpose of regulating dietary supplements.

Uncovering the Regulator: Exploring the Therapeutic Goods Administration (TGA)
The Therapeutic Goods Administration (TGA) is the Australian Government agency responsible for regulating therapeutic goods including medicines, medic

Discover What is ISO/TS 16949 & IATF 16949 & Why it Matters
ISO/TS 16949 and IATF 16949 are two important standards recognized globally for automotive-specific Quality Management Systems (QMS).

What is 21 CFR Part 606? An Essential Guide to FDA Compliance
The FDA 21 CFR Part 606 regulations are designed to regulate the safety of both food and drug products.

Unpacking the Regulations: Understand 21 CFR 1271
21 CFR 1271 is a regulation set forth by the US Department of Health and Human Services (HHS) that addresses the manufacturing, processing, and labeli

Unveiling the Mysteries of 21 CFR Part 820 & ISO 13485
The 21 CFR Part 820 is a quality system regulation (QSR) issued by the United States Food and Drug Administration (FDA) which governs the design, manu

21 CFR Part 11: Open vs Closed Systems – What’s the Difference?
The 21st Century Cures Act (21 CFR Part 11) is a US Food and Drug Administration (FDA) regulation on the security and integrity of electronic records

Unraveling Regulations: 21 CFR Part 11 vs EU Annex 11
In today’s digital age, organizations must be equipped to manage electronic records in a consistent, secure manner. In order to achieve this obj

Compliance with HITRUST: What It Is and Why It Matters
HITRUST certification is a highly-regarded IT security and compliance certification program. HITRUST stands for Health Information Trust Alliance, and

Discover What Healthcare Compliance Means & How to Implement It
Healthcare compliance is the practice of making sure that organizations follow all laws, regulations, and policies related to the healthcare industry.

Master the Basics: Unlocking the Power of eMandates
An eMandate is an electronic authorization for direct debit payments. The system is designed to streamline the payment process, reducing administrativ

Unlock HIPAA Compliance: The Complete Guide
The Health Insurance Portability and Accountability Act (HIPAA) is a standard which all healthcare providers, organizations, and insurance companies m

USP & FDA to Make Critical Updates to Good Storage & Distribution Practices
This blog post will discuss the proposed updates to Good Storage and Distribution Practices from the United States Pharmacopeia (USP) and the Food and

Need to Respond to FDA 483s? Here’s What You Need to Know
FDA 483 observations (or “Form 483s”) are issued by the Food and Drug Administration (FDA) when findings of a GMP (Good Manufacturing Prac

Discover What GxP Compliance Audits Are & Who Performs Them
GxP independent compliance audits are processes developed to ensure that companies adhere to certain standards of quality, safety, and regulatory comp

Ready for Clinical Trial GCP Audits? Here’s What You Need to Know
A Good Clinical Practice (GCP) audit is an important part of clinical trials that ensures that the methods used and data collected are meeting certain

An In-depth Look at Integrity & Reliability in Bioequivalence Studies
Bioequivalence studies are an important process for ensuring that the pharmaceutical products produced by drug companies meet regulatory standards, su

FDA Announces New Reg. for Easy-to-Understand Medication Info for Patients
When it comes to taking medications, it’s important to understand what you’re consuming and the possible risks associated with doing so.

Get Ready for the International Recognition Framework (IRF): Understand Your Commitments
The International Recognition Framework (IRF) is an important tool for organizations as it sets out requirements for compliance with international sta

Take a Peek Into Common Critical Findings Found in Compliance Auditing
Compliance auditing is an important component of regulatory compliance. It involves a thorough review of procedures, processes, and records to ensure

Discover What the Drug Supply Chain Security Act (DSCSA) Means for You!
The Drug Supply Chain Security Act (DSCSA) is a law of the United States that was passed in 2013 to create a national system for the regulation and co

Unlocking AML Guidelines: IRDAI AML/CFT Compliance in Insurance
In the ever-evolving landscape of the insurance industry, staying compliant with regulatory guidelines is paramount.

Demystifying SEBI’s 2023 Cloud Framework Guidelines
In the rapidly evolving landscape of financial markets, the Securities and Exchange Board of India (SEBI) stands as a formidable guardian of inte

RBI’s Latest Regulations: Safeguarding Digital Lending Against Malpractices
In today’s fast-paced digital age, the financial landscape is undergoing a significant transformation.

Unlock Compliance: Uncover GAMP 5 for GxP Compliant Computer Systems
GAMP 5 stands for Good Automated Manufacturing Practice (GAMP) 5, an important concept in the GMP industries.

Unlock the Benefits of FDA ISO 13485 and 21 CFR Part 820 Harmonization
The U.S. Food and Drug Administration (FDA) is the governing body that regulates medical device manufacturers.

Unlock Your Auditing Career: 7 Essential Skills for GMP Internal Auditors
Internal auditing plays an important role in Good Manufacturing Practices (GMP). GMP Internal Auditors must have certain essential skills to perform t

Unlock the Keys to Good Manufacturing Practices: A Guide
GMP stands for Good Manufacturing Practices and it is a rigorous set of regulations that must be followed by companies who are involved in the product

Navigate CSV Traffic Jams with Validation 4.0: How to Automate Compliant Document Delivery
Validation 4.0 is a new system designed to help teams deliver documents and content quickly while still maintaining regulatory compliance.

Secure Your ChatGPT & AI Tech – Uncover Security Threats & Mitigate Risks
ChatGPT is an AI chatbot technology developed by OpenAI, a San Francisco-based company. It uses natural language processing to generate human-like res

Uncover the Fundamentals: A CISOs Guide to FedRAMP
FedRAMP is an acronym for the Federal Risk and Authorization Management Program. Devised under a collaborative effort between the government, industry

Discover the Top Compliance Challenges Facing Fast-Growing Businesses
Compliance is an important issue for all companies, but it can be especially challenging for fast-growing companies.

Unpacking the Differences: ISO 9001 vs ISO 13485 for Medical Devices
ISO 9000 is an international quality management system (QMS) standard, and ISO 13485 is a QMS Standard specifically designed for medical devices.

Unlock the Secrets of 21 CFR Part 11 Compliance for Medical Device Mfgs
21 CFR Part 11 is a set of regulations developed by the United States Food and Drug Administration (FDA) to enhance the protection of electronic recor

Master FDA 21 CFR 820 Compliance: The 6 Biggest Risk Areas in 2023
The regulation and compliance of FDA 21 CFR 820 is an important element for any organization or company that produces, markets, stores, distributes, o

Pass Your ISO 13485 Audit: All You Need to Know!
ISO 13485 is a global quality management system (QMS) standard used by medical device manufacturers.

Uncover the Benefits & Challenges of Clinical Laboratory Improvement Amendments (CLIA).
Clinical Laboratory Improvement Amendments (CLIA) is a set of laws and regulations that are designed to ensure that all laboratory testing is performe

Transition Your Medical Devices to New European MDR: Are You Ready?
Welcome to our guide on transitioning your medical devices to Europe’s New Medical Device Regulation (MDR)! This guide will provide an overview

Conquer the Top 6 Quality & Compliance Oversights in Pharma
The success of any business in the pharmaceutical industry relies heavily on its ability to navigate the ever-changing regulations that are required f

Equip Your Drug Launch with Nitrosamine Regulations: Essential Guide
This guide is designed to help organizations understand and comply with the nitrosamine regulations related to marketing applications for new drug pro

Learn to Uncover Value from Clinical Trials in Australia
Australia is home to world-class medical research, and clinical trials are a major part of this. Clinical trials are federally regulated studies that

Unlock the Potential of Clinical Research: Why Decentralized Clinical Trials Are the Future
Decentralized clinical trials (DCTs) are a new approach to conducting clinical research that shifts the traditional model in significant ways.

Unlock Safety and Security with GVP: A Quick Guide
Good pharmacovigilance practices (GVPs) are procedures used to protect the safety of patients taking medications.

Uncovering FDA’s Nitrosamine Impurity Regulations for NDSRIs
NDSRI stands for Non-selective Dopamine Reuptake Inhibitor and is a type of medication used to treat depression and other mental health conditions.

Data Integrity: Harness Its Power to Seamlessly Transition to Pharma 4.0
In the current digital age, data integrity has become increasingly important as organizations strive to transition into a more automated and efficient

Unlock Quality Improvement In Pharma W/ Smart Goals
A smart goal is an acronym for Specific, Measurable, Achievable, Relevant and Time-bound. It is an effective method used by many organisations to ensu

Uncover the UDI System: Why it Matters
The UDI System, short for Unique Device Identification System, is a system used to identify medical devices through the use of labels, barcodes, or ot

Solving Sustainability Challenges: Pharma Industry and Sustainability by Design
The pharmaceutical industry plays a critical role in the health and wellbeing of millions of people all over the world.

Gain Insight: Design Verification & Validation for Medical Devices
Design verification and design validation are processes used in the production of medical devices.

Overcome Critical Challenges with QbD: How to Implement QbD Successfully
Quality by Design (QbD) is an approach to drug development and manufacturing that focuses on optimizing the quality of the final product by understand

Discover the Pros & Cons of Pharmaceutical Contract Manufacturing
Pharmaceutical contract manufacturing helps businesses meet the increasingly complex demands of the industry.

Mastering Clinical Trials: Guarantee Quality with 4 Essential Phases
Clinical trials are essential for medical research and have proven to be a key element in advancing medical treatments and developing new drugs.

Unlock Pharmaceutical QA Mastery with 4 Easy Steps!
Today, the world of pharmaceuticals is becoming increasingly complex. With global health concerns rising to the forefront, pharmaceutical companies ar

Step-by-Step Guide to Starting a Pharmaceutical Company
Today, the world of pharmaceuticals is becoming increasingly complex. With global health concerns rising to the forefront, pharmaceutical companies ar

Unlock the Secrets of cGMP: 7 Experts Explain
Good Manufacturing Practice (GMP) is a set of guidelines, established by the World Health Organization (WHO), that focus on pharmaceutical manufacturi

Reduce Risk & Reap Benefits: Everything You Need to Know About Design Controls for Med Devices
Design Controls are an essential part of designing and developing any medical device. They help to ensure the safety, effectiveness and quality of a p

Dive Deeper Into Risk Management for Medical Device Startups
Risk management is an essential part of any business, and medical device startups are no exception. Without effective risk management, medical device

21 CFR Part 11 eSignature Compliance Checklist Here’s What You Need to Know About FDA Regulations (Updated)
Keeping sensitive data and private information secure is extremely important as well as complex

21 CFR Part 11 Compliance Checklist to Follow
21 CFR Part 11 outlines the FDA’s requirements for the integrity, quality, and compliance of digital documents and also signatures.

21 CFR Part 11 and Good Documentation Practices in Pharmaceutical Industries
It has been said that in the pharmaceutical industry, “If it isn’t documented, it didn’t happen.