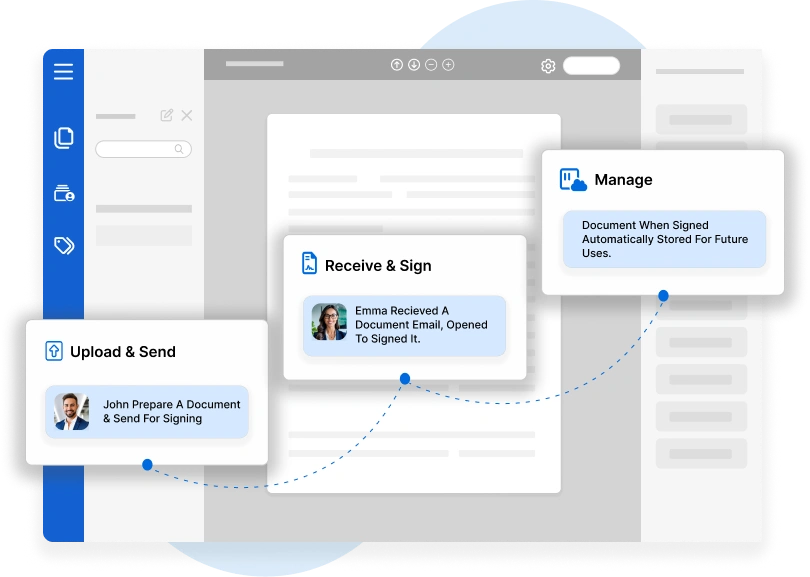
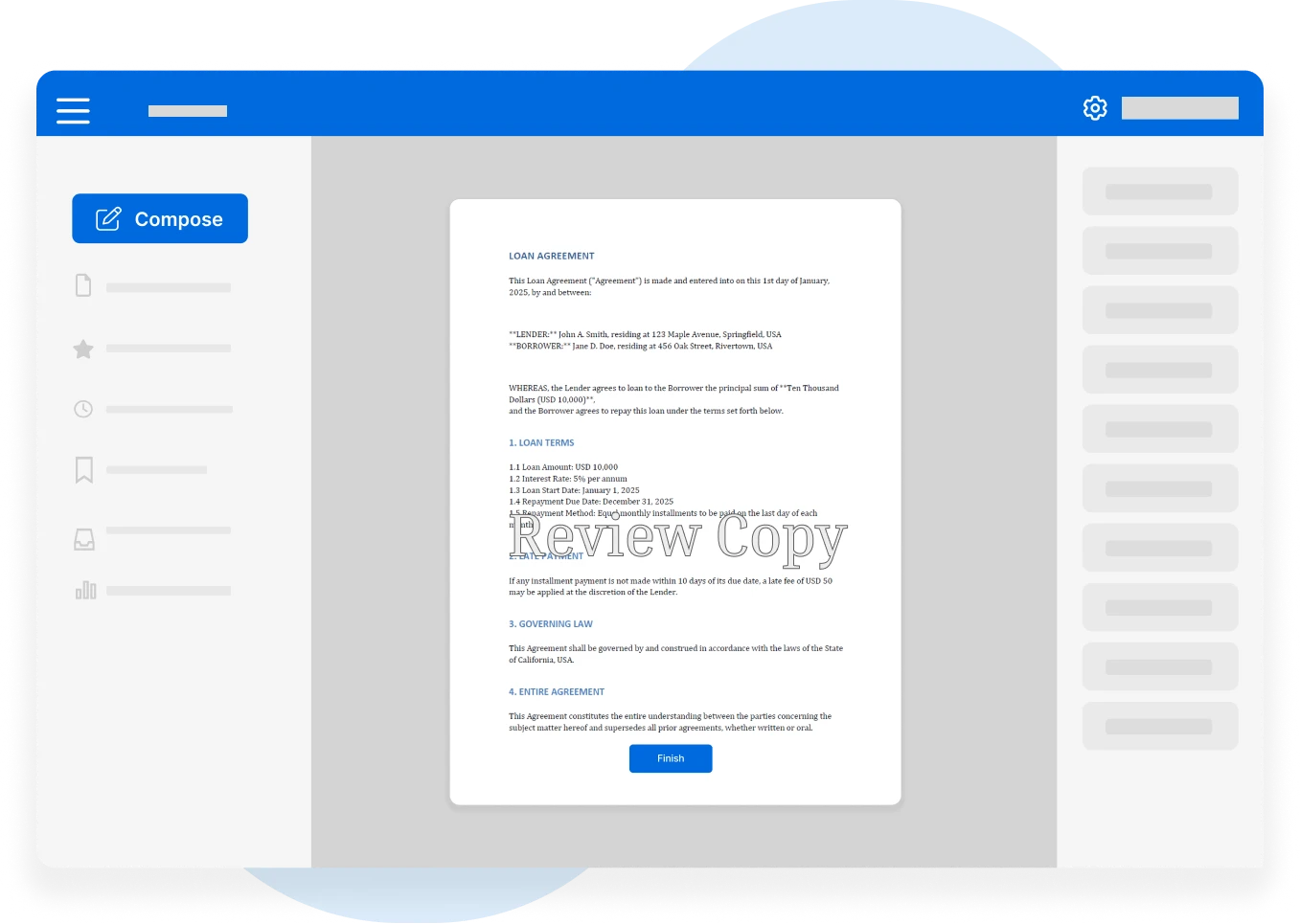
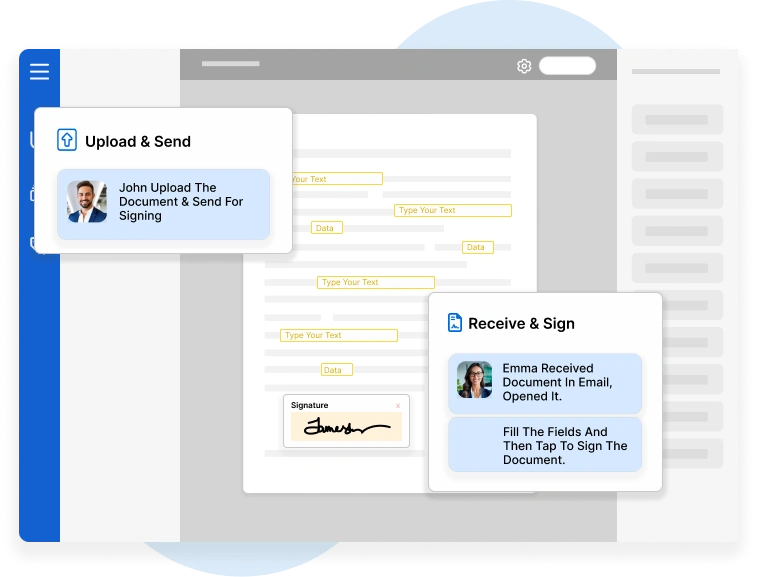
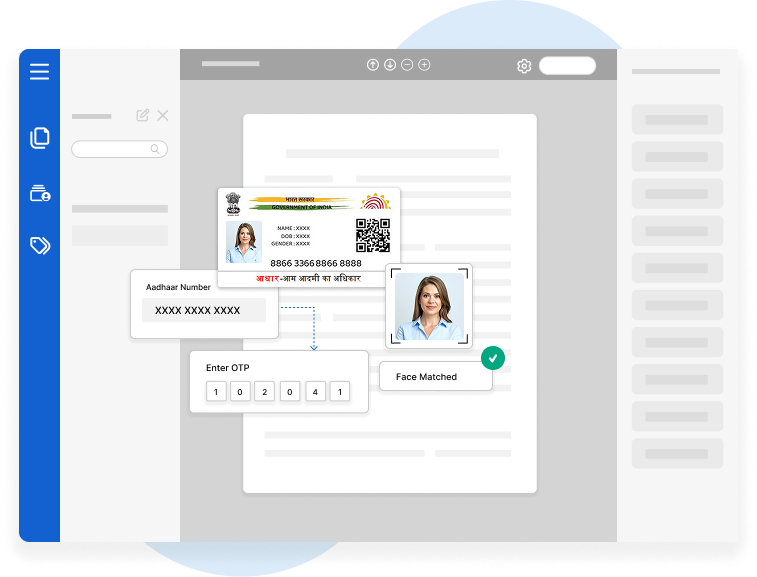
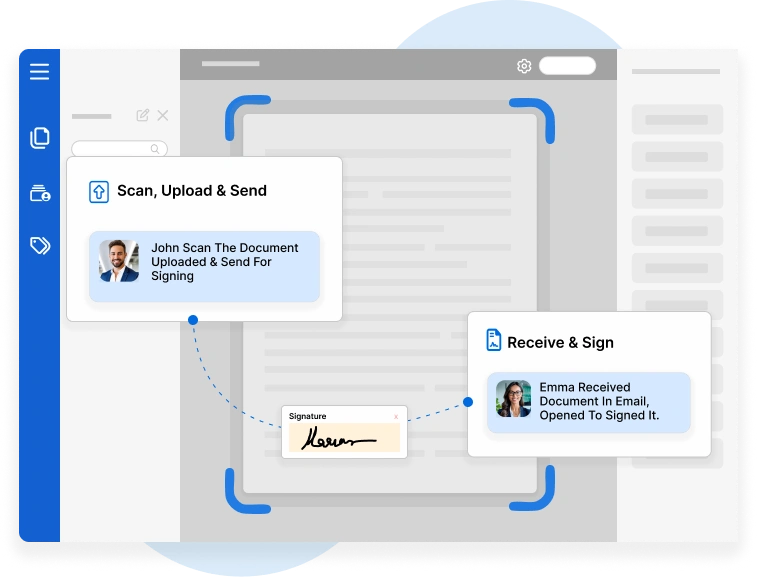
In the highly regulated world of pharmaceuticals, biotechnology…

In today’s digital era, maintaining data integrity is a top priority for FDA…

The life sciences industry operates within a highly regulated environment…

Pfizer achieved 75% efficiency by transforming its documentation…

Indraprastha Apollo Hospitals Implements digital signatures…

HMSI achieved 84% reduction in paper helping the company achieve with…
Enabling CROs, pharma, healthcare and lifesciences industries with disruptive eSignature

MSB Docs announced as a winner in MG Motor’s as a Platform Innovation Programme