- Email us: sales@msbdocs.com

Founded in 1896 in Basel, Switzerland, Roche is a global leader in healthcare with expertise in both pharmaceuticals and diagnostics. The company develops innovative medicines and diagnostic solutions that improve millions of lives worldwide. With its unique strength in personalized healthcare, Roche is advancing research and innovation to deliver effective therapies, reduce treatment costs, and improve patient outcomes.
Roche faced rising costs and inefficiencies in clinical trials, R&D, and operations due to highly regulated processes and manual documentation. Lengthy approval cycles and reliance on printing, scanning, and faxing slowed productivity and increased expenses.


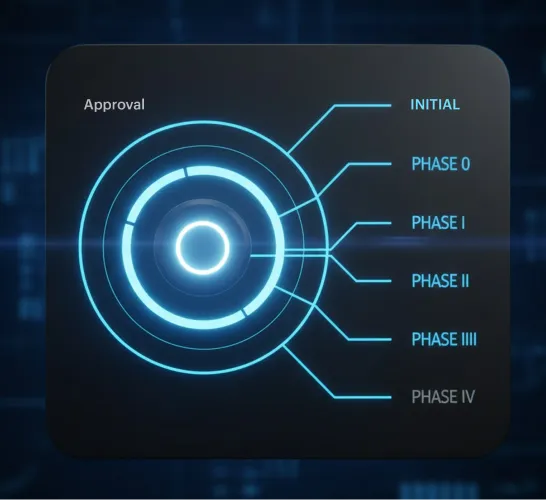



MSB Docs transformed Roche’s manual signing processes into a secure, digital workflow. With eSignatures, SSO, and Team Rooms, the company reduced cycle times from days to hours, cut costs, improved compliance, and enabled researchers to focus on research instead of paperwork.
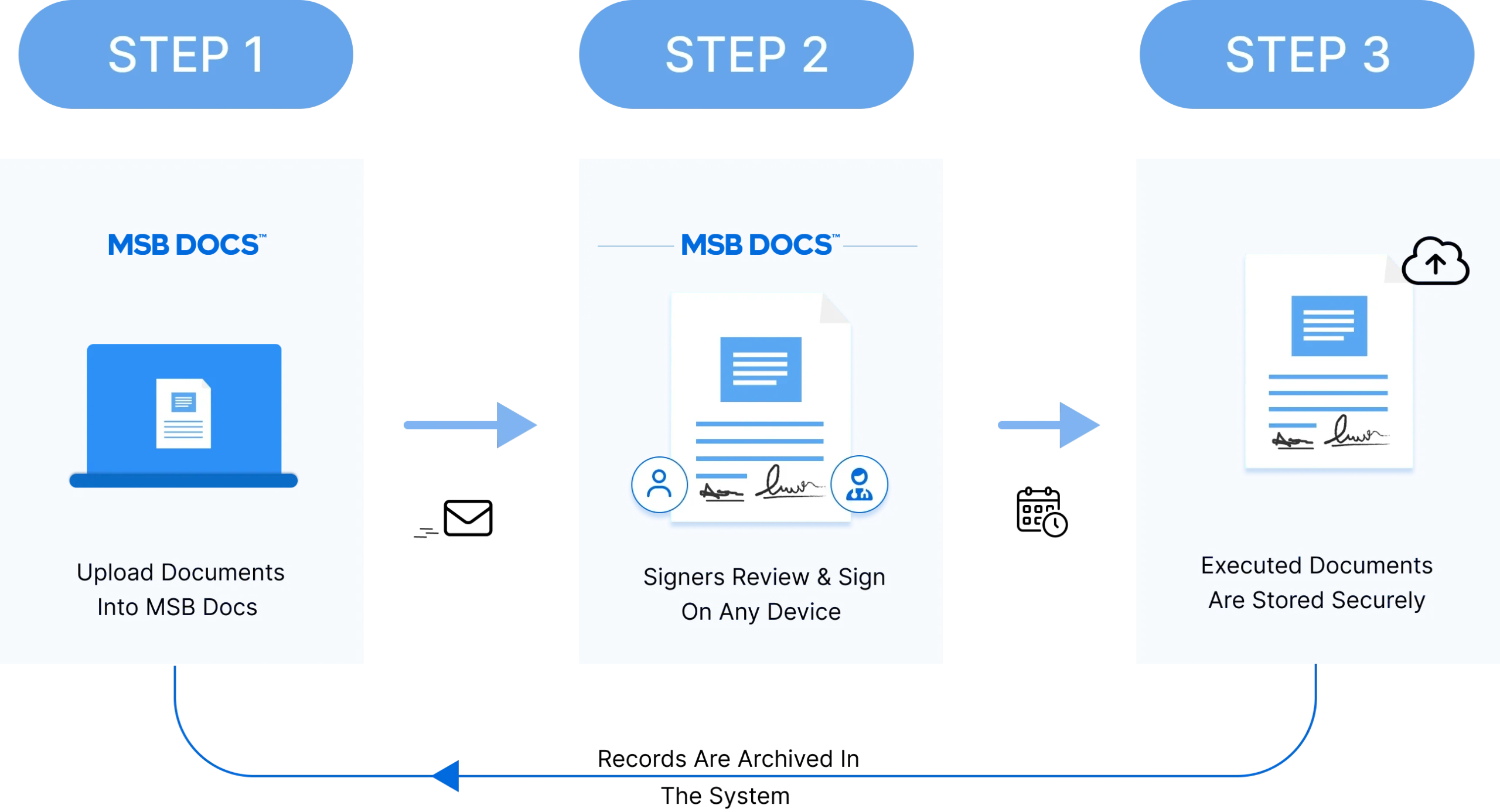
With MSB Docs, Roche achieved a faster, more secure, and fully digital document process. Cycle times were reduced from days to hours, improving workforce productivity and collaboration. Chemists could focus on research instead of paperwork, boosting lab efficiency and output. Instant eSignatures with date-time stamps strengthened compliance and patent protection, while the detailed audit trail supported regulatory needs and audits. By eliminating paper-based workflows, Roche realized significant cost savings and ensured long-term digital compliance with the standards of 21 CFR Part 11.